Written by Calla Reed
Hold on to your masks! Our current pandemic may only be the beginning. Despite its contested location of origin, it is well documented that COVID-19, also known as betacoronavirus SARS-CoV-2, originated in a bat population and mutated to “jump” from bats to humans.
Similarly, early strains of HIV originated in the West African gorilla population, then mutated and created unique virus strains in the human population. Same with Ebola, which has mutated multiple times from either bats or primates to cause recurring disease outbreaks in humans. Even the Bubonic Plague still exists naturally in animal populations and threatens to become viable in humans.
Zoonotic diseases, infectious diseases that exist naturally in an animal population and mutate to become viable in the human population upon close contact, have been the cause of history’s most impactful pandemics. Due to climate change, increased deforestation, and exponential rates of urbanization, humans will be in closer and more consistent contact with wildlife. This trend significantly increases the possibility of frequent epidemic outbreaks, with some estimating they could occur about 5-6 times a year.
Zoonosis, or zoonotic disease transmission is the largest source of infectious disease. Over 200 known cases of zoonotic disease transmission have been recorded and 75% of emerging infectious diseases are zoonotic in origin. Some of these diseases, such as certain HIV strains in the West African lowland gorilla population, are as dangerous and deadly to the animal population as the mutated HIV strains are to humans. However, this is not usually the case: “many animal species harbor pathogens—some of which they live with and are largely unaffected by” (Sarah Bowden, quantitative ecologist, Emory University). There are two components that contribute to zoonotic disease transmission between humans and animals: human-animal contact and the microbiology of viruses.
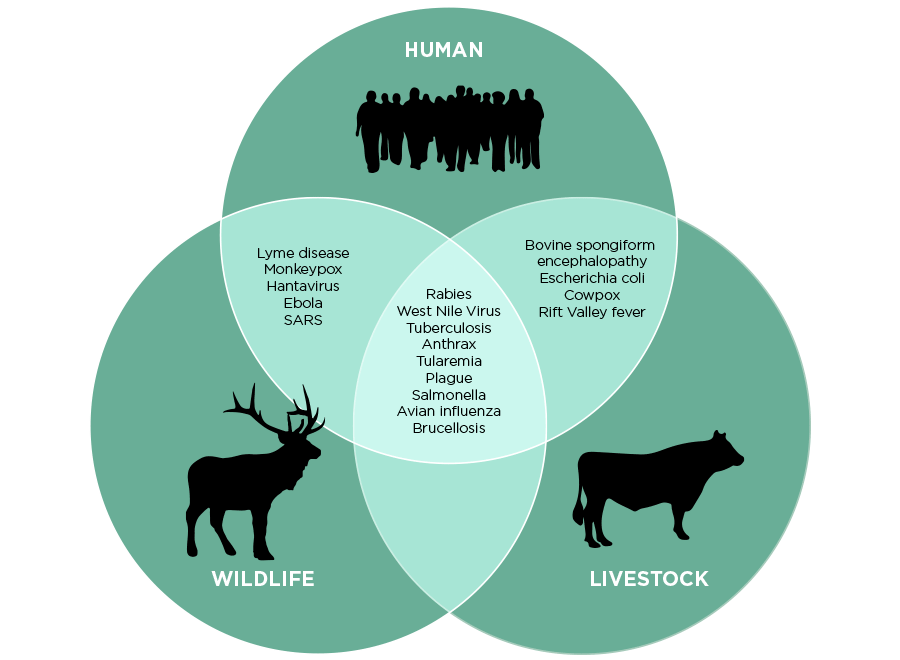
The contact component requires that a human be in close direct or indirect contact with the pathogen in order to become infected. This can occur through direct contact with the infected animal, indirect contact with the habitat of the animal (through contaminated food or water) or through a third-party vector (such as Plasmodium falciparum in mosquitoes, the cause of malaria).

The microbiological component has to do with the viability of the virus once the human has come into contact with the pathogen. Some RNA viruses lack proofreading mechanisms in their RNA polymerases and have the ability to swap sections of their genomes with viruses of the same type. Thus, they may come into contact with a human but fail to become viable in that individual’s cells due to errors in the viral genetic variants. For zoonotic diseases that have been around for a significant amount of time, the human population may have some natural adaptation to protect against viability. For example, the sickle cell’s phenotype’s malformed red blood cells prevent the pathogen Plasmodium falciparum from successfully replicating.
However, due to their lack of proofreading activity, RNA viruses are able to create a significantly larger pool of genetic variants with higher rates of mutation while hovering around a theoretical “error threshold” in order to maintain the integrity of their genetic information. This allows many RNA viruses to quickly and easily determine which mutations are the most beneficial; these variants are then used for further replication and adaptation.
So, despite harboring many errors in their genomes, RNA viruses are able to mutate and replicate themselves quickly, making it difficult to both contain viruses in one population (whether human or animal) and eradicate them completely (only two infectious diseases, smallpox and rinderpest, have been eradicated). Faster rates of mutation result in more errors in the RNA variants and make these infectious diseases more contagious.
Both contact and viability have to be established in order for a virus to mutate from an animal population to a human population. While viability remains unchanged, the increased contact between humans with animals will increase the probability of zoonotic disease transmission taking place. According to the World Health Organization (WHO), increased urbanization will result in more people living directly adjacent to wilderness areas or in semi-urban areas with higher numbers of wild animals at risk of disease. With both climate change and deforestation rampantly destroying the natural habitats of nearly all species of wildlife, the human population is expected to have an increased number of epidemic outbreaks, especially since “the majority of emerging infectious diseases are coming from wildlife” (Thomas Gillespie, disease ecologist, Emory University).
On a positive note, virologists and wildlife experts are already working together to address this issue. On an individual level, experts are examining the three planes of zoonosis: the risk landscape (the spatial geography of where an infection is most likely), different animal reservoirs (which animals are common carriers of pathogens susceptible to zoonotic disease transmission), and different pathogens (what diseases are common, or endemic, to the area). On an international level, the One Health approach, a collaborative effort between WHO, the Food and Agriculture Organization of the United States (FAO), and the World Organization for Animal Health (OIE) has created the Global Early Warning System for Major Animal Diseases (GLEWS). This coalition assists in the surveillance, reporting, and localized containment of targeted zoonotic diseases. Additionally, the United Nations Summit on Biodiversity will be addressing this issue on September 30th, making climate change’s effect on zoonotic disease transmission a forefront issue in preventing the next epidemic. The lessons learned from the current pandemic and its pervasive consequences will hopefully help us battle future epidemics that occur due to the negative impacts of climate change. While the current pandemic may only be the beginning, hopefully the lethality of future epidemics will decrease due to our knowledge gained concerning zoonotic disease transmission.


I have been trying to understand why zoonotic disease transmission could increase for awhile…thank ya for explaining so weIl and sharing! I’m thinking: how to cure the animals of the diseases (so they won’t pass to humans and also animals will be healthier and humans will be fulfilled caring for beings❤️), and also how to protect and share habitat for animal and human life and coexistence.
Hi Beth! Thank you so much for taking the time to read my article! I’m so glad it was helpful. Curing the animals of these viruses and pathogens has recently been a significant part of conservation efforts, especially concerning the Western lowland gorilla population in certain parts of Cameroon, where the population is becoming endangered due to unique strains of HIV. That’s why the World Health Organization adopted the One Health Approach, a unique approach where animal health, public health, and environmental experts collaborate on conservation among climate change. Feel free to check out their website here: https://www.who.int/news-room/q-a-detail/one-health . Thanks again and have a great day!